Which Lewis Structure Best Describes the Carbonate Ion
Which of the following Lewis structures is the best structure for the ammonia molecule. As an CaCO 3 can be given.
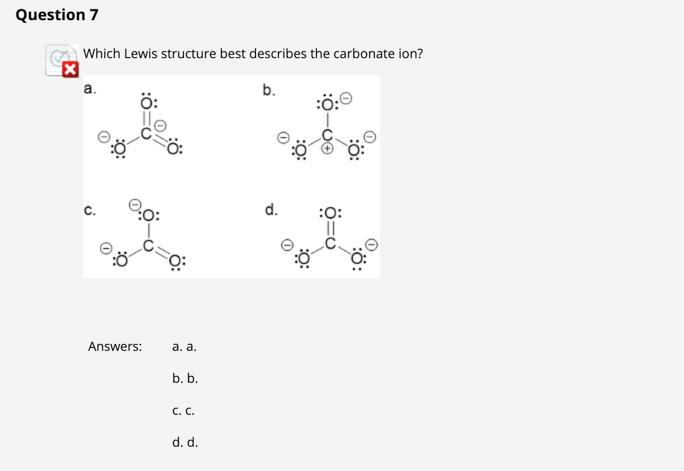
Solved Question 7 Which Lewis Structure Best Describes The Chegg Com
How many valence electrons does the carbonate ion have.
. Chemistry questions and answers. A HCl b CF 4 c PCl 3 d PF 5. A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure Carbonate ionFor the CO3 2- structure use the periodic table to find the total nu.
Add that all up. Metal carbonates generally decompose on heating liberating carbon dioxide and leaving behind an oxide of the metal. Because the CO3 2- ion has a charge of negative 2 we need to put brackets around our Lewis structure and put that negative 2 outside so everyone knows that it is an.
Toothpastes containing sodium hydrogen carbonate sodium bicarbonate and hydrogen peroxide are widely used. Asked Aug 2 2019 in Chemistry by GradStudent. Add electrons for negative charge.
Co32- lewis structure molecular geometry. Including any valid resonance structures. Except hydrogen Go to Electronegativities chart.
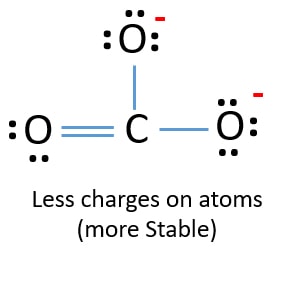
The correct Lewis structure for this ion has one carbonoxygen double bond and two carbonoxygen single bonds. Describe one resonance structure of the carbonate ion. The carbon atom in pure structure still needs to share electrons to satisfy the.
Lewis structure of NO 2-ion is drawn in this tutorial. Carbonate ion CO 3 2-Carbonate ion has a -2 charge. There are three σ bonds and π bond around carbon atom in the Lewis structure of CO.
Which statement concerning this structure is incorrect Two electrons are being shared between the carbon atom and the oxygen atom. Salt of the carbonic acid carbonates space widely supplied in a variety of industrial and domestic applications. Describe one resonance structure of the carbonate ion.
The Lewis structure of formaldehyde is shown below. Draw the Lewis Structure of carbonate ion CO32-. Please include the resonance structures and the formal charges on each atom.
The Carbonate CO 2 3 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Count the total valence electrons in the molecule. This is probably from the electrons of sodium calcium or whatever salt resulted in a cation that the donated electrons to the carbonate anion.
Determine the formal charge of each element in the following. Now we are going to learn how to draw this lewis structure. Theres one last thing we need to do.
Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. Carbonate has 24 electrons 2 of them responsible for the -2 charge probably electrons from calcium sodium or whatever salt resulted in a cation that donated electrons to the carbonate anion. Each of the singly bonded oxygen atoms bears a formal charge of 1 and all other atoms are neutral.
Place the least electronegative atom in the center. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule with resonance forms where appropriate. So -1 plus -1 that does match up with what we have for the carbonate ion here.
Decide on the arrangement of atom. Metal carbonate compounds are common in the world. This structure is incompatible with the observed symmetry of the ion which implies that the three bonds are the same length and that the three oxygen atoms are equivalent.
Few of these encompass glass and also ceramic creation food preservation and also iron extraction. No difference between structures a b с Water has a relatively high specific heat compared to metals. 70 More Lewis Dot Structures.
There are three different possible resonance structures from carbonate. CO 3 2-Lewis structure. Since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet rule.
Our first attempt in drawing carbonate ions lewis dot structure results in the structure as represented below. The CO 3 2-The CO 3 2-The CO 3 2-The CO 3 2-. Which Lewis structure although acceptable least describes the carbonate ion.
Because of that this is the best Lewis structure for CO3 2-. Question 7 Which Lewis structure best describes the carbonate ion. If a 20-g piece of a nonreactive metal at 80C is dropped into 20 g of cool water at 20C what will be the final temperature of water.
Oxygen has six we have 3 Oxygens and this negative 2 means we have an extra two valence electrons. Our first attempt at drawing the lewis dot structure of the carbonate ion results in the structure shown below. How many valence electrons does the acetate ion have.
Draw the Lewis structure for CO32- including any valid resonance structures. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure. The Lewis structure of the carbonate ion has two long single bonds to negative oxygen atoms and one short double bond to a neutral oxygen atom.
Carbon has 4 valence electrons. Lets do the CO3 2- Lewis structure. Carbonates are one of the most frequently found and also discussed ionic reality in the ar of chemistry.
Carbon is the least electronegative put that at the center. Lewis Dot of the Carbonate Ion. The answer is 3 May i recommend a video Lets consider the Lewis structure of the carbonate ion CO32.
Carbonate ion holds 24 electrons where 2 of them are responsible for a charge of -2. Lewis Structure for NO 2-Nitrite ion. Draw the Lewis structure for CO 3 2-Select which option answers top prompt best.
4 plus 18 plus 2. Draw the Lewis Structure b. Subtract electrons for positive charge.

Lewis Structure For Co32 Carbonate Ion
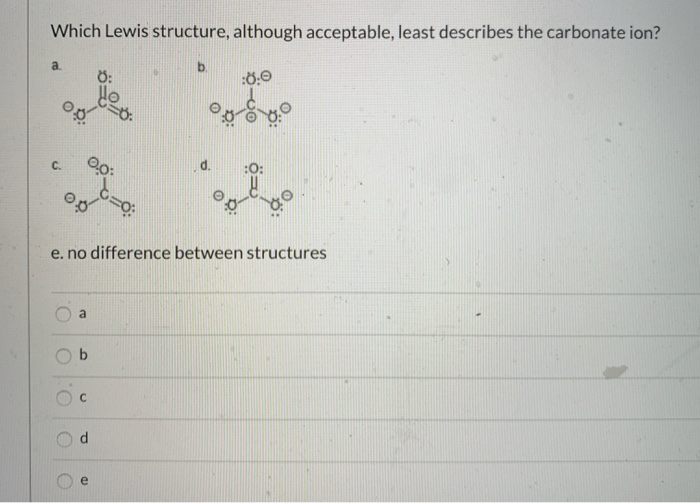
Solved Which Lewis Structure Although Acceptable Least Chegg Com
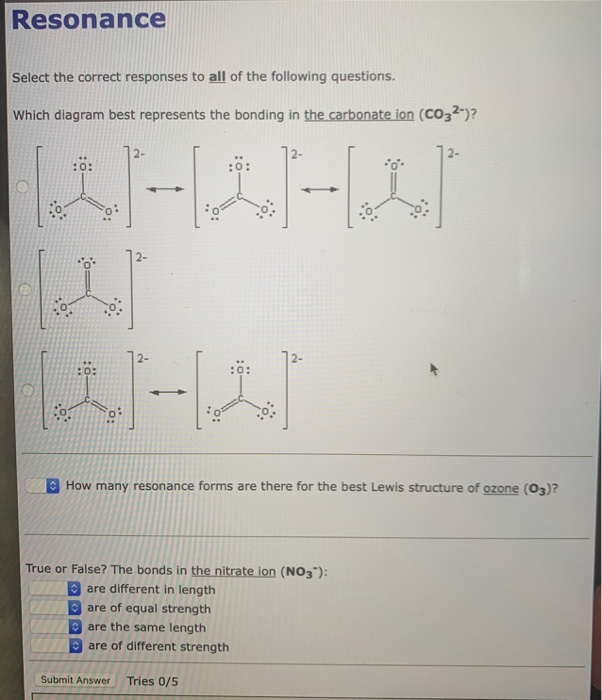
Solved Resonance Select The Correct Responses To All Of The Chegg Com
No comments for "Which Lewis Structure Best Describes the Carbonate Ion"
Post a Comment